Abstract
BACKGROUND Ibrutinib is a Bruton's Tyrosine Kinase Inhibitor indicated in the treatment of several B-cell malignancies: Chronic Lymphocytic Leukemia (CLL), Mantle Cell Lymphoma (MCL) and Waldenström Macroglobulinemia (WM). The number and the characteristics of patients treated by Ibrutinib in France in real life settings are unknown and need to be determined, as well as treatment pattern in real-life settings.
METHODS All patients affiliated with the general scheme (around 80% of the French population) with a first dispensing of ibrutinib from August 1, 2017 (date of reimbursement in France) to December 31, 2020 were identified in the French national health insurance database (SNDS). Indications for which ibrutinib was supposed to be used were defined by an algorithm based on ICD-10 codes to identify the disease (CLL, MCL or WM), and on codes of previous and concomitant dispensed drugs to understand treatment line (first line -L1- or second or later line -L2+). Patients’ characteristics were described overall and by disease. A discontinuation was defined as temporary if the duration of Ibrutinib discontinuation was 6 months or less, and as permanent otherwise. Time to permanent discontinuation of Ibrutinib (including death) and time to next treatment (including death) were estimated and plotted using Kaplan Meier curves, by disease. Hospitalizations for a cardiovascular (CV) or bleeding event (atrial fibrillation, hypertension, bleeding, cardiac arrest, paroxysmal tachycardia, heart failure) were described in patients with CLL.
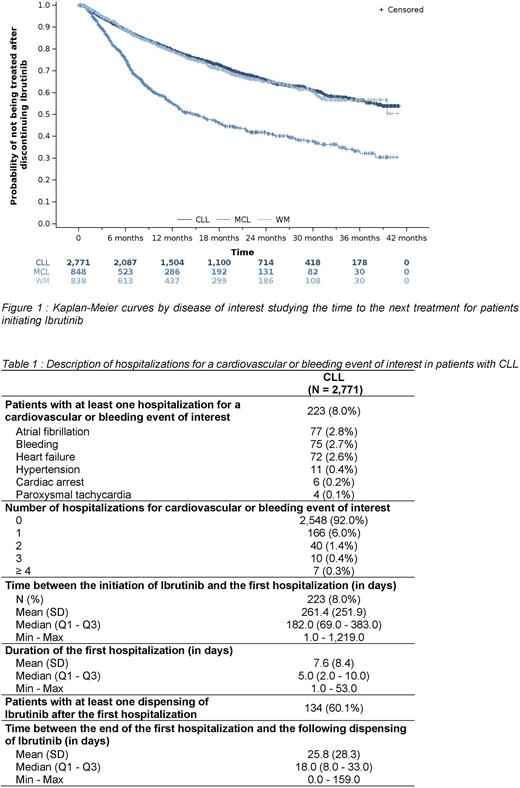
RESULTS The study population consisted of 6,083 patients who initiated ibrutinib from August 1, 2017 to December 31, 2020. Among them, 45.6% had CLL, 13.9% had MCL, 13.8% had WM and 26.7% were unclassified because of the absence of specific codes associated with CLL, MCL or WM. The median duration of follow-up of patients with CLL, MCL and WM was 16.2, 9.5 and 15.2 months, respectively. Patients were elderly (median age of 71.4 years; 64% of patients over 70 years old) and mostly men (63.0%). At ibrutinib initiation, 68.3% of patients had a Charlson comorbidity index of 5 or more. In fact, 47.6% of patients had at least one CV disease in the year before the initiation and 21.5% had at least one solid tumor. Most patients used Ibrutinib as a second or later line (80.2%, 94.2% and 94.5% of patients with CLL, MCL and WM, respectively). The probability of permanently discontinuing Ibrutinib or dying at 12 months was 27% (including 10.7% death), 52% (including 27.8% death) and 29% (including 10.7% death) in patients with CLL, MCL and WM, respectively. The median time to permanent discontinuation or death was 11.2 months in patients with MCL and was not reached for patients with CLL and WM. The probability of treatment switch or dying at 12 months was 21%, 45% and 21% in patients with CLL, MCL and WM, respectively. The median time to switch or death was 14.9 months in patients with MCL and was not reached for patients with CLL and WM (Figure 1). In the 2,771 patients with CLL, 8.0% (223) were hospitalized for a CV or bleeding event of interest over the follow-up (median time of onset of 6 months after ibrutinib initiation), among whom 60.1% (134) had another dispensing of Ibrutinib after this hospitalization (Table 1). The main reasons for hospitalization were atrial fibrillation (2.8%, 77), bleeding (2.7%, 75) and heart failure (2.6%, 72).
CONCLUSION This is one of the largest cohorts of Ibrutinib patients in the world. This study provides interesting and useful data on the real-life use of Ibrutinib. As all claims database studies, clinical outcomes data are lacking. However, the size and the representativeness of the dataset facilitates reliable trends for results. Indeed, the characteristics of the population were consistent with those expected. This study supports the use of Ibrutinib in real-life setting in elderly patients with CV comorbidities. Moreover, this study highlights that few CLL patients experienced a hospitalization for CV or bleeding event of interest, among which more than half took again Ibrutinib after their hospitalization.
Disclosures
Deslandes:Janssen: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.